Answer:
Option A,B,D
Explanation:
(a) For a first-order reaction. the concentration of reactant remaining after time t is given by $[A]=[A]_{0}e^{-kt}$. Therefore, the concentration of reactant decreases exponentially with time
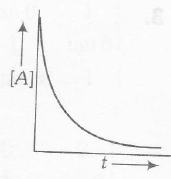
(b) Rise in temperature increase rate constant (k) and therefore decrease half-life $(t_{1/2})$ as
$t_{1/2}=\frac{ln 2}{k}$
(d) For a first order reaction. if 100 moles of reactant is taken initially, after n half-lives reactant remaining is given by percentage
$A= 100(\frac{1}{2})^{n}=100(\frac{1}{2})^{8}=0.3906$
$\Rightarrow $ A reacted =100-0.3906=99.6%
(c) Half-life of first order reaction is independent of initial concentration