Answer:
Option A,C
Explanation:
(a) Entropy is a state function, change tn entropy in a cyclic process is zero'
Therefore.
$\triangle S_{X \rightarrow Y}+\triangle S_{Y \rightarrow Z}+\triangle S_{Z \rightarrow X}$=0
$\Rightarrow$ $-\triangle S_{Z \rightarrow X}=\triangle S_{X \rightarrow Y}+\triangle S_{Y \rightarrow Z}$
=$\triangle S _{X \rightarrow Z}$
Analysis of options (b) and (c)
Work is a non-stable function, it does depend on the Path followed.
$W_{Y\rightarrow Z}=0$ as $\triangle V=0$ Therefore $W_{X\rightarrow Y \rightarrow Z}=W_{X\rightarrow Y}$
Also work is the area under the curve on p-V diagram.
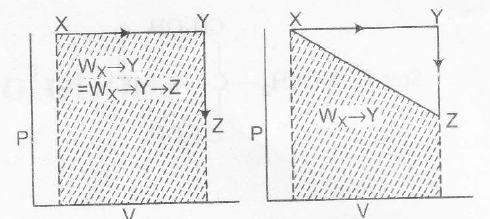
As shown
$ W_{X \rightarrow Y}+W_{Y\rightarrow Z}=W_{X\rightarrow Y}= W_{X\rightarrow Y \rightarrow Z}=W_{X\rightarrow Y} $
but not equal to $W_{X \rightarrow Z}$