Answer:
Option B
Explanation:
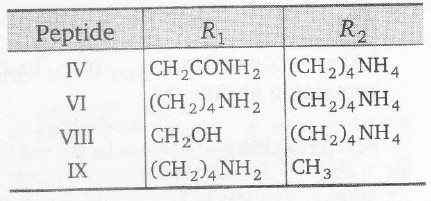
The amino acid remains completely in Zwitterionic form at its isoelectric point. Amino acids with the additional acidic group have their isoelectric pH less than 7.0 and increasing pH above the isoelectric point makes them anionic. On the other hand, amino acids with the additional basic groups have their isoelectric pH greater than 7.0 and decreasing pH below the isoelectric point (by adding acid solution) makes them cationic. The given peptide with following R1 and R2 are basic, will remain Protonated (cationic) at PH = 7.0.