Answer:
Option D
Explanation:
In $XeO_{2}F_{2}$ the bonding arrangement around the central atom Xe is
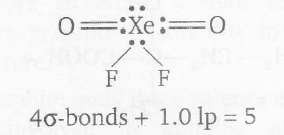
$\Rightarrow$ Hybridization of Xe=$sp^{3}d$
$sp^{3}d$ hybridization corresponds to trigonal bipyramidal geometry. Also, in trigonal bipyramidal geometry, lone pairs remain present on equatorial positions in order to give less electronic repulsion.
.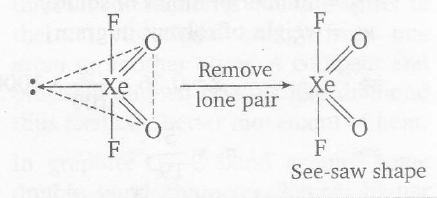
Note According to Bent's ru1e, the more electronegative atoms must be present on the axial position. Hence, F is kept on axial positions.