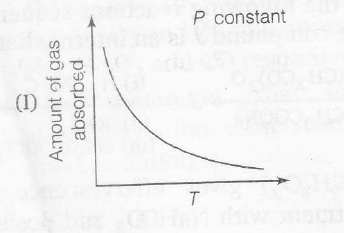
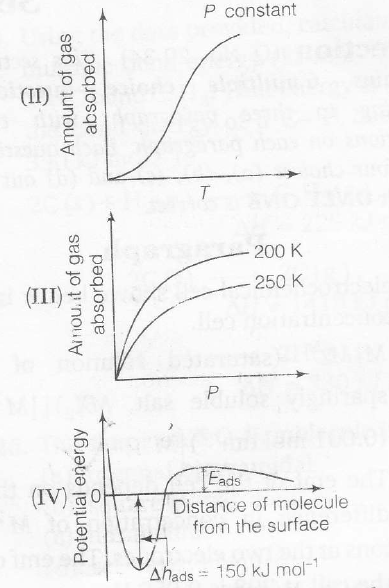
The given graph/data I, II, III and IV , represent general trends observed for different Physisorption and chemisorption processes under mild conditions of temperature and pressure' Which of the following choice(s) about I, II, III and IV is (are) correct?