Answer:
Option A,B,C,D
Explanation:
Plan A specially said to have aromatic character if
(a) ring is planar
(b) there is complete delocalisation of $\pi$ electrons
(c) Huckel rule i.e, (4n+2) rule is followed
where n is the number of rings (4n+2)=$\pi$ electron delocalised.
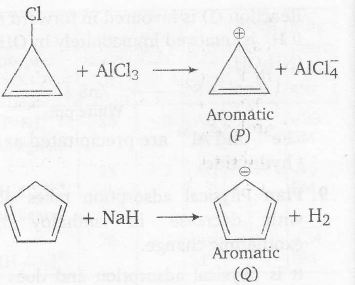
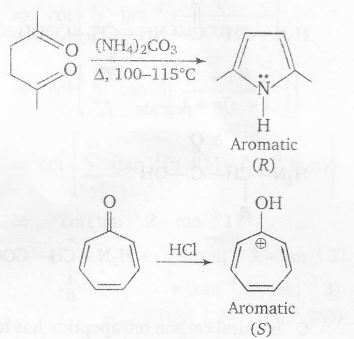
n (4n+2) $\pi$ electrons
P - 0 2 2
Q - 1 6 6( Including lone pair)
R - 1 6 6( including lone pair on N)
S - 1 6 6
In all cases there is complete delocalisation of $\pi$ -electrons