Answer:
Option A
Explanation:
Plan Reactant is cyclic anhydride and changes to dicarboxylic acid on hydrolysis
Also, there is decarboxylation on heating if there is keto group w.r.t -COOH group. Ozonolysis cleaves $(C=C) $bond and $H_{2}O_{2}$ oxidises -CHO to -COOH group
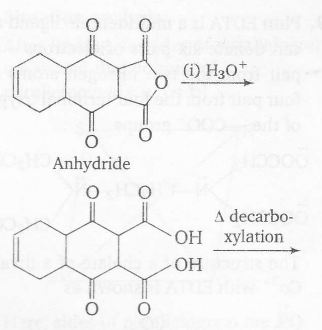
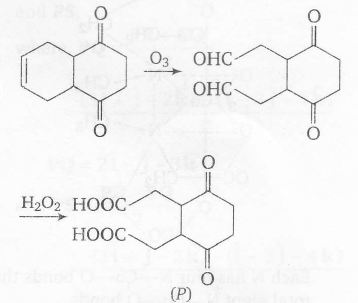
Thus, number of -COOH group in P=2