Answer:
Option A
Explanation:
Plan A peptide linkage is hydrolysed to two free amino acids.
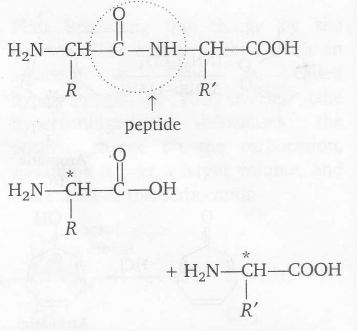
C' is a chiral carbon tetrapeptide that has four amino acids joined by three peptide linkage.
-COOH group is on the alanine part, thus it is a fixed C-terminal position in each combination.
Glycine is optically inactive thus it can't be on the N-terminal side.
Thus, possible combinations are
Phe-Gly-Val-Ala
Phe-Val-Gly-Ala
Val-Gly-Phe-Ala
Val-Phe-Gly-Ala
Thus, in all four possible combinations``