Answer:
Option A,C
Explanation:
$L=3(\frac{h}{2\pi})$
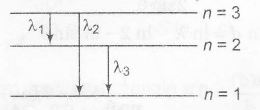
$\therefore$ n=3 as $L=3(\frac{h}{2\pi})$
$r_{n}\propto\frac{n^{2}}{z}$
r3= 4.5 a0 $\therefore$ z=2
$\frac{1}{\lambda_{1}}=Rz^{2}\left(\frac{1}{2^{2}}-\frac{1}{3^{2}}\right)=4R\left(\frac{1}{4}-\frac{1}{9}\right)$
$\therefore$ $\lambda_{1}=\frac{9}{5R}$
$\frac{1}{\lambda_{2}}=Rz^{2}\left(\frac{1}{1^{2}}-\frac{1}{3^{2}}\right)=4R\left(1-\frac{1}{9}\right)$
$\Rightarrow$ $\lambda_{2}=\frac{9}{32R}$
$\frac{1}{\lambda_{3}}=Rz^{2}\left(\frac{1}{1^{2}}-\frac{1}{2^{2}}\right)=4R\left(1-\frac{1}{4}\right)$
$\Rightarrow$ $\lambda_{3}=\frac{1}{3R}$