Answer:
Option A
Explanation:
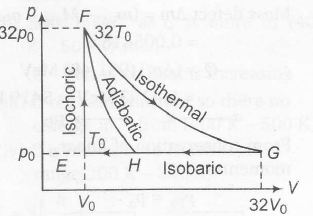
in F→ G work done in isothermal process is
$nRT ln\left(\frac{V_{f}}{V_{i}}\right)=32p_{0}V_{0}ln\left(\frac{32V_{0}}{V_{0}}\right)$
$=32p_{0}V_{0}ln2^{5}=160p_{0}V_{0}ln 2$
$In G\rightarrow E, \triangle W=p_{0}\triangle V=p_{0}(31V_{0})=31p_{0}V_{0}$
In G $\rightarrow$ H , work done is less than 31 p0V0 , i.e, 24 p0V0
In F $\rightarrow$ H workdone is 36 p0V0