Answer:
Option A,C,D
Explanation:
Plan Due to resonance, bond lengths between two atoms are equal. Species is said to be diamagnetic if all electrons are paired process is endothermic if it takes place with the absorption of heat
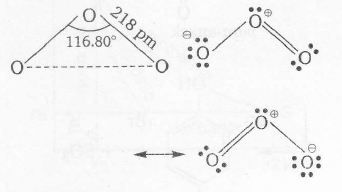
bent molecule
all electrons paired thus, diamagnetic
2O3 → 3O2 , $\triangle $ H° = -142 kJ mol-1
Exothermic
Thus, (b) is incorrect
(a,c, d ) are correct