Answer:
Option C
Explanation:
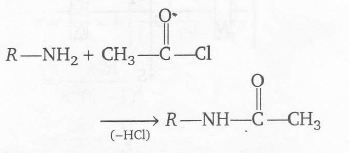
since each -COCH3 group displace one H atom in the reaction of one mole of
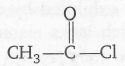 with one -NH2 group the molecular mass increases with 42 unit. Since the mass increases by (390-180)=210
with one -NH2 group the molecular mass increases with 42 unit. Since the mass increases by (390-180)=210
hence the number of -NH2 group is 210/42=5