Answer:
Option E
Explanation:
(a) ONCl= 8+7=17=32 e-
ONO- =8+7+8+1=24 e- (correct)
(b) 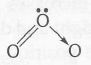
central O atom is sp2 hybridised with 1 lone pair, so bent shape (correct)
(c) In solid state, ozone is violet -black ozone does not exist in solid state(correct)
(d) O3 has no unpaired electron, so diamagnetic (correct)
* No option is incorrect