Answer:
Option B
Explanation:
Plan. This product can be solved by using concept of conformational analysis of given organic compound. To solve the question draw the stable conformational structures of organic compound and determine the net resultant dipole moment
The confirmation of the given compound are as follows
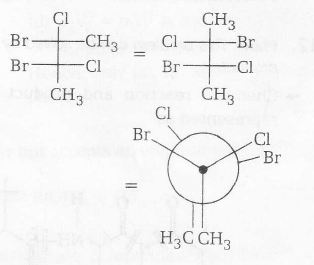
Stable conformer (with $\mu\neq0$ )
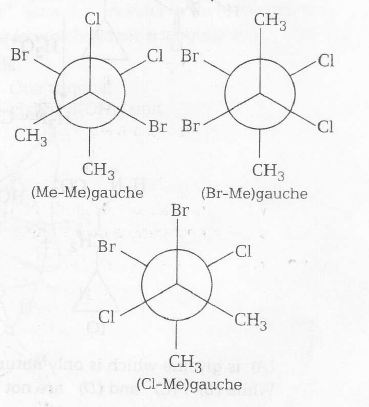
These three have non-zero dipole moment due to the non-cancellation of all dipole moment created by C-Cl and C-Br bond