Answer:
Option C
Explanation:
Plan This problem can be solved by using the concept of stability of carbocation and SN1 reaction when two phenyl groups are replaced by two para methoxy group, carbocation formed will be more stable. As the stability of carbocation formed increases, rate of acidic hydrolysis increases.
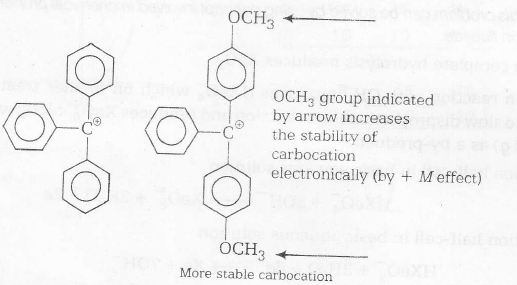
Hence, (c) is the correct, choice