Answer:
Option C
Explanation:
Plan This problem includes basic concept of bonding. It can be solved by using the concept of molecular orbital theory
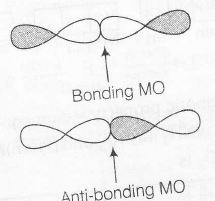
Any orbital has two-phase +ve and -ve. In the following diagram +ve phase is shown by darkening the lobes and -ve by without darkening the lobes

When two same phase overlap with each other it forms bonding molecular orbital otherwise antibonding
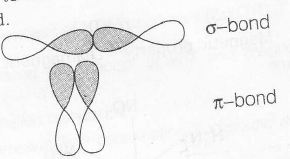
Axial overlapping leads to the formation of σ -bond and sideways overlapping leads to the formation of $\pi$- bond
On the basis of above two concepts correct matching can be done as shown below
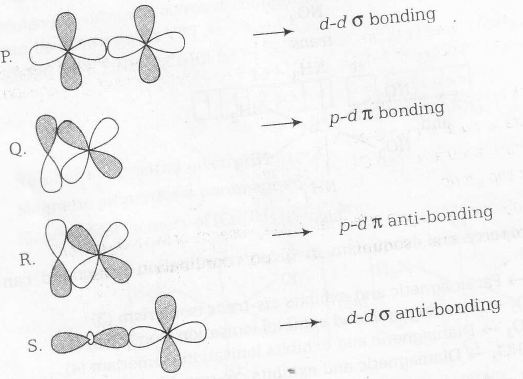
P → 2, Q → 3, R→ 1, S→ 4
Hence ,(c) is the correct option