Answer:
Option A
Explanation:
In this question, the system is accelerating horizontally i.e, no component of acceleration in vertical direction. Hence, the pressure in the vertical direction will remain unaffected
i.e, p1 =p0+ρgh
Again, we have to use the concept that the pressure in the same level will be same.
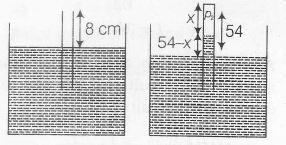
For air trapped in tube, p1V1=p2V2
$p_{1}=p_{atm}=\rho g76$
V1= A.8
[A= area of cross-section]
$p_{2}=p_{atm}-\rho g(54-x)$
$=\rho g(22+x)$
V2 =A.x
$\rho g 76\times 8A=\rho g(22+x)Ax$
$x^{2}+22x-78\times 8=0$
$\Rightarrow$ $x=16 cm$