Answer:
Option C
Explanation:
In CsCl,Cl- lie at corners of simple cube and Cs+ at the body centre . Hence , along the body diagonal , Cs+ and Cl- touch each other so,
$r_{Cs^{+}}+r_{Cl^{-}}=2r$
Calculation of $\tau$
In $\triangle EDF$
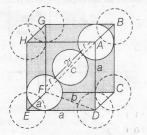
Body centred cubic unit cell
$FD=b=\sqrt{a^{2}+a^{2}}=\sqrt{2}a$
In $\triangle AFD$,
$c^{2}=a^{2}+b^{2}$
$=a^{2}+(\sqrt{2}a)^{2}$
$=a^{2}+2a^{2}$
$c^{2}=3a^{2}$
$c=\sqrt{3}a$
As $\triangle AFD$ is an equilateral triangle,
$\therefore$ $\sqrt{3}a=4r$
$\Rightarrow r =\frac{\sqrt{3}a}{4}$
Hence, $r_{Cs^{+}}+r_{Cl^{-}}=2r$
= $2\times\frac{\sqrt{3}}{4}a=\frac{\sqrt{3}}{2}a$