Answer:
Option C
Explanation:
Decreasing order of strength of oxoacids
$ HClO_{4} > HClO_{3}> HClO_{2} >HOCl$
The reason, Consider the structure of conjugate bases of each oxyacid of chlorine.
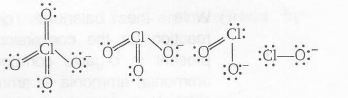
The negative charge is more delocalised on ClO4- due to resonance. Hence, ClO4- is more stable (and less basic).
Hence, we can say as the number of oxygen atoms around Cl-atom increases as oxidation number of Cl-atom increases and thus the ability of loose the H+ increases