Answer:
Option B
Explanation:
Arrange the complex formed by different ligands L1, L2, L3 and L4, according to wavelength of their absorbed light, then use the following relation to answer the question.
Ligand field strength $\propto$ Energy of light absorbed
$\propto$ 1/ wavelength of light absorbed
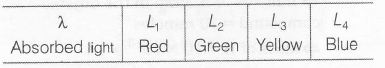
Wavelength of absorbed light decreases.
$\therefore$ Increasing order of energy of wavelength absorbed reflect greater extent of crystal -field splitting. Hence higher field strength of the ligand.
Energy Blue (L4) > green (L2)
> yellow (L3)
> red (L1)
$\therefore$
L4>L2>L3>L1 , in field strength of ligands.