Answer:
Option B
Explanation:
When S is donor atom of SCN- , it produces weak ligand field and forms high spin complex as
$\left[Fe \left(SCN\right)_{6}\right]^{3-};Fe^{3+}(3d^{5})=$
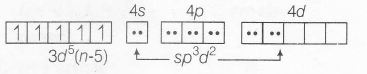
Spin only magnetic moment
$(\mu_{s})=\sqrt{5(5+2)}BM=\sqrt{35}BM$
In case of CN- ligand , carbon is the donor atom, it produces strong field and forms low spin complex as
$\left[Fe(CN)_{6}\right]^{3-}:Fe^{3+}(3d^{5})$
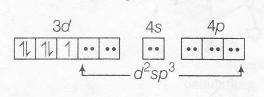
Spin only magnetic moment $(\mu_{s})=\sqrt{1(1+2)}BM$
$=\sqrt{3}BM$
Hence, difference in spin only magnetic moment
$=\sqrt{35}-\sqrt{3}=4BM$