Answer:
Option B
Explanation:
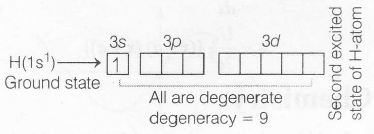
In one electron (hydrogenic) system, all orbitals of a shell remains degenerate, hence in second excited state, the degeneracy of H-atom is nine
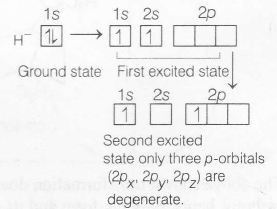
In case of many electrons system, different orbitals of a shell are non-degenerate, Hence,