Answer:
Option B.C
Explanation:
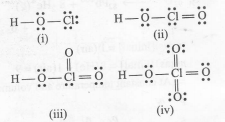
(a)number of Cl-=O bonds in(ii) and (iii) together is three.Hence, wrong.
(b) number of lone pair of on Cl in(ii) and (iii) together in three. Hence , correct
(c)In (iv) , Cl is $sp^{3}$ -hybridised. Hence, correct.
(d)amongest (i) and (iv) , the strongest acid is(iv). Hence , wrong