Answer:
Option A
Explanation:
Alkene in which different groups are attached with the double-bonded carbon atoms, exhibit geometrical isomerism.
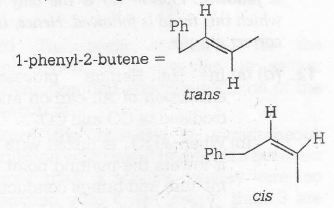
It will show geometrical isomerism,
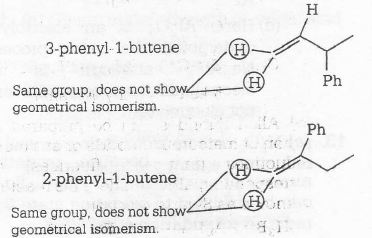
1,1- diphenyl-1- propane being an alkane (saturated compound) does not show geometrical isomerism.
Time Saving Technique.
We do not need the check all options, but it should remind that double-bonded compounds show geometrical isomerism. Thus (d) is eliminated, (i.e, propane). Now, eliminate the terminal alkene and get the correct response.