Answer:
Option B
Explanation:
According to Freundlich adsorption isotherm, $\frac{x}{m}=kp^\frac{1}{n}$
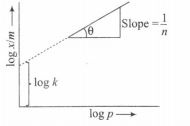
On taking the logarithm of both sides, we get
$\log \frac{x}{m}=\log k + \log p^\frac{1}{n}$
$\log \frac{x}{m}= \log k + \frac{1}{n}\log p$
y=c+mx
$y=\log \frac{x}{m}$ c=intercept = log k
$m= slope=\frac{1}{n}$
and x= log p