Answer:
Option B,D
Explanation:
The graph shown indicates that there is a positive deviation because the observed vapor pressure of L is greater than the ideal pressure.
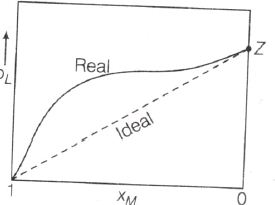
Since, the deviation is positive, the intermolecular force between L and M is smaller than the same in pure L and pure M.
Also as XL→ 1, XM → 0. The real curve approaching the ideal curve where Raoult's law will be obeyed.