Answer:
Option (B,C)
Explanation:
(i) Statement (a) The total number of valence shell electrons at metal centre in Fe (CO)5 or Ni(CO)4 is 8 instead of 16 as shown below.
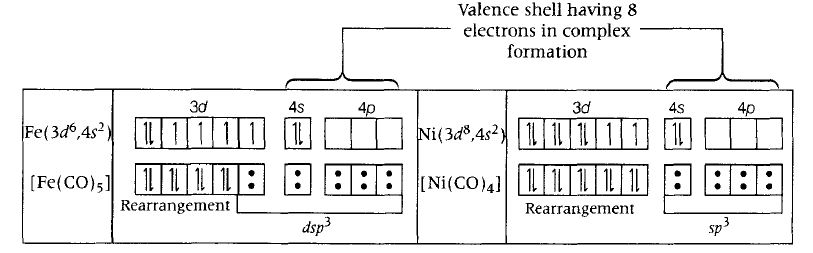
Hence, this statement is incorrect
(ii) Statement (b) Carbonyl complexes are predominantly low spin complexes due to strong ligand fields. Hence, this statement is correct
(iii) Statement (c) For central metal lowering of oxidation state results to increase in electron density on it. This in turn results to increase in the extent of synergic bonding. Thus, we can say "metal carbonyl bond strengthens when oxidation state of the metal is lowered".
Hence, it is a correct statement.
(IV) Statement (d) Increase in positive charge on metal (i.e., increase in oxidation state) results to decrease in synergic bonding strength.
This, in turn, makes the C-O bond stronger instead of weaker. Hence this statement is also incorrect.