Answer:
Option C
Explanation:
The unit cell of initial structure of ionic solid MX looks like
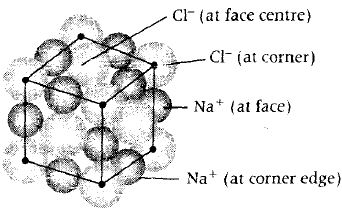
In NaCl type of solids cations (Na+ ) occupy the octahedral voids white anions (Cl- ) occupy the face centre positions.
However , as per the demand of problem the position of cations and anions are swapped.
We also know that (for 1 unit cell)
(A) Total number of atoms at FCC= 4
(B) Total number of octahedral voids =4'
( as no. of atoms at FCC= No. of octahedral voids)
Now taking the conditions one by one.
(i) If we remove all the anions except the central one than number of left anions.
=4-3=1
(ii) If we replace all the face centred cations by anions than effective number of cations will be = 4-3=1
Likewise effective number of anions will be=1+3=4
(iii) If we remove all the corner cations then effective number of cations will be 1-1=0
(IV) If we replace central anion with cation then effective number of cations will be 0+1=1
Likewise effective number of anions will be 4-1=3
Thus, as the final outcome, total number of cations present in Z after fulfilling all the four sequential instructions=1
Likewise, total number of anions =3
Hence, the value of
Number of anions/ Number of cations = $\frac{3}{1}=3$