Answer:
Option A
Explanation:
Equation of cell reaction according to the cell notation given, is
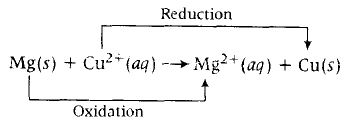
Given $E_{cell}^0=2.7V$. T= 300K
with [ Mg 2+ (aq)]= 1 M and [Cu 2+ (aq)]= 1 M and n=2
Further . Ecell =2.67 V
with [Cu 2- (aq)]= 1M and [Mg 2+ (aq)]=xM
and $\frac{F}{R}=11500KV^{-1}$
where F= Faraday constant, R= gas constant
From the formula,
$E_{cell}=E_{cell}^{0}-\frac{RT}{nF}ln\frac{[Mg^{2+}(aq)]}{[Cu^{2+}(aq)]}$
After putting the given values
$2.67= 2.70- \frac{RT}{2F}ln\frac{x}{1}$
or $2.67= 2.70- \frac{RT}{2F}ln x$
$-0.03= \frac{-R\times 300}{2F}lnx$
Or $ln x= \frac{0.003\times 2}{300}\times\frac{F}{R}$
= $\frac{0.003\times 2 \times11500}{300}=2.30$
So, ln x= 2.30
or x=10 (as given ln(10)=2.30)