Answer:
Option A
Explanation:
The structures of various molecules given in problem are discussed below -
1. N2O3 It is the tautomeric mixture of following two structures-
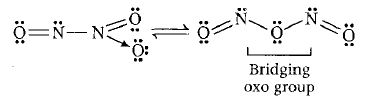
Conclusion 1 bridging oxo group is present in the compound.
2. N2O3 It has following structure.
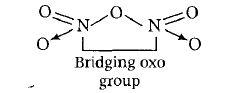
Conclusion 1 bridging oxo group is present in the compound.
where, p1 = initial pressure, p1= total pressure
y= number of gaseous products per mole of reactant
3. P4 O6
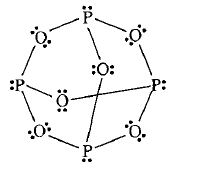
Conclusion 6 bridging oxo groups are present in the compound.
4. P4 O7

Conclusion 6 bridging oxo groups are present in the compound.
5. H4P2O5
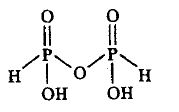
Conclusion 1 bridging oxo groups is present in the compound.
6. H5P3O10
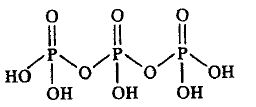
Conclusion 2 bridging oxo groups are present in the compound.
7. H2S2O3
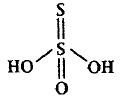
Conclusion This compound doesnot contain any bridging oxo group.
8. H2S2O5
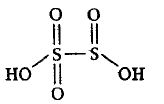
Conclusion This compound also doesnot contain any bridging oxo group.