Answer:
Option C
Explanation:
Given that , i= 100 amp. also 27.66 g of diborane ( B2H6)
Molecular mass of B2H6 = $10.8\times 2+6 =27.6$
Number of moles of B2H6 in 27.66 g= $\frac{Given mass}{Molecular mass}=\frac{27.66}{27.6}=1$
Now consider the equation
$B_{2}H_{6}+3O_{2}\rightarrow B_{2}O_{3}+3H_{2}O$
From the equation we can interpret that 3 moles of oxygen is required to burn 1 mole ( i.e 27.6 g) B2H6 completely.
Also consider the electrolysis reaction of water i.e
$H_{2}O\rightleftharpoons 2H^{+}+O$
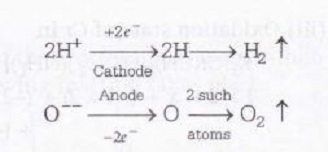
From the above equation it can be easily interpreted that in electrolysis of water for the production of 1 mole of oxygen from 1 mole of H2O at anode 4 moles electrons are required.
Likewise for the production of 3 moles of O2 12 ( 3× 4) moles of electrons will be needed.
So, the total amount of charge required to produce 3 moles of oxygen will be 12 × F or 12 × 96500
We know Q= it
So, 12 × 96500 = 100 × t in seconds
or $\frac{12\times 96500}{100\times 3600}=t$ in hours = 3.2 hours