Answer:
Option C
Explanation:
Key Idea Among the given compounds the basic nature depends upon their tendency to donate electron pair
Among the given compounds in
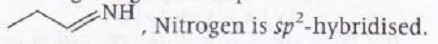
This marginally increases the electronegativity of nitrogen which in turn decreases the electron donation tendency of nitrogen. Thus making compound least basic.
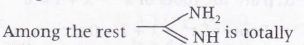
differnent from others as in this compound lone pair of one nitrogen are in conjugation with π bond

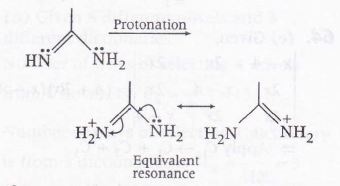
This equivalent resonance is cation
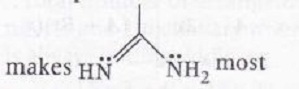
basic among all.
Categorisation is very simple between rest two as
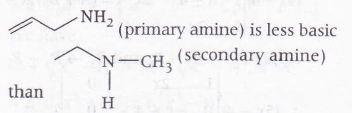
Hence, the correct order is
( II)<( I)<( IV)<( III) i.e option ( c) is correct.