Answer:
Option D
Explanation:
Effective Atomic Number (EAN) can be calculated by the following relation.
EAN= Z(atomic number of the metal) -number of electrons lost in the ion formation + number of electrons gained from the donor atoms of the ligands.
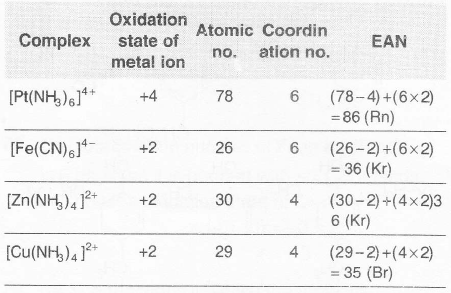
$[Cu(NH_3)_4]^{2+}$ is an exception of EAN rule.