Answer:
Option B
Explanation:
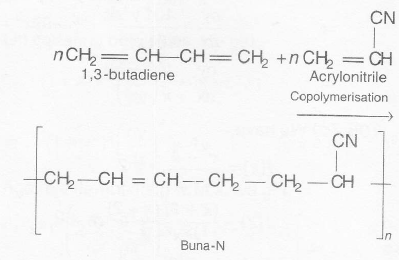
Dextron is a copolymer of glycolic acid and lactic acid consisting of ester linkages that makes it biodegradable
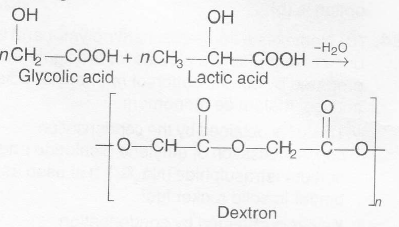
Nylon-2-nylon -6 is a copolymer of glycine
(H2N-CH2-COOH) and aminocaproic acid (H2N(CH2)5 COOH) and is biodegradable in nature
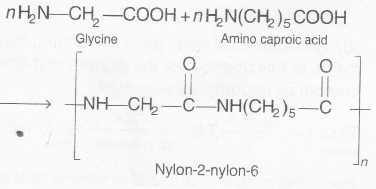
. PHBV (poly-$\beta$- hydroxybutyrate- co- $\beta$-hydroxy valerate ) is obtained by copolymerisation of 3-hydroxybutyric acid and 3-hydroxy pentanoic acid.
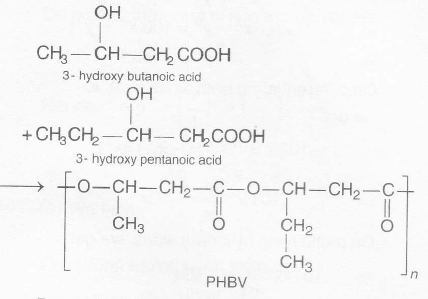
. Buna-N is obtained by copolymerisation of 1,3 butadiene and acrylonitrile in the presence of peroxide catalyst.