Answer:
Option B
Explanation:
(a) Dipole moment of HBr

(b) Dipole moment of acetone
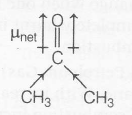
due to methyl group, (ERG) lies in direction of oxygen therefore, net dipole moment increases
$\mu _{net}=2.69 D$
(c) Dipole moment of $H_{2}S$
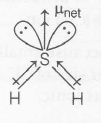
$\mu _{net}=0.97 D$
(d) Dipole moment of $COCl_{2}$
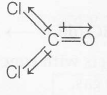
due of Cl atom net dipole moment decreases.
$\mu _{net}=0.69 D$
Hence, acetone has highest dipole moment