Answer:
Option C
Explanation:
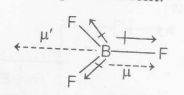
$BF_{3}$, has zero dipole moment
In $NH_{3}$, N is more electronegative than H. So, nitrogen pulls the electron from H towards itself and the direction of moment is same due to N-H bond as that of the lone pair of electrons on nitrogen
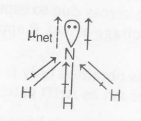
$\mu _{net}=1.46 D$
In $NF_{3}$ , F is more electronegative than N, All F atoms pull the electrons towards themselves and resultant dipole moment is opposite to the direction of that of the lone pair at N atom, Hence , net dipole moment decreases.
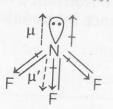
$\mu _{net}=0.24 D$
Therefore, dipole ment of $NH_{3}$ is greater than $NF_{3}$ . Hence , order of dipole moment is
$NH_{3} > NF_{3} > BF_{3}$