Answer:
Option C
Explanation:
If a tertiary alkyl halide is used, an alkene is the only reaction product and no ether is formed. For example, the reaction of CH3ONa with (CH3)3C-Br gives exclusively 2-methylpropene.
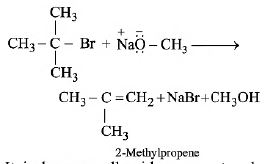
It is because alkoxides are not only nucleophiles but strong bases as well. They react with alkyl halides leading to elimination reactions