Answer:
Option B
Explanation:
For path iaf, Q = 50 cal W = 20 cal
By the first law of thermodynamics,
ΔU = Q - W = 50 - 20 = 30 cal.
For path ibf
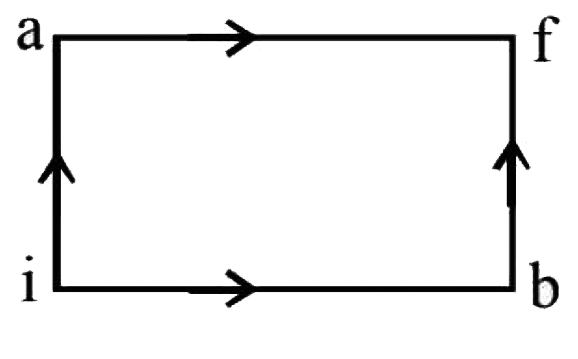
Q' = 36 cal
W' =?
Or, W' = Q' - ΔU'
Since the change in internal energy does not depend on the path,
therefore ΔU' = 30cal
.'. W' = Q' - ΔU' = 36 - 30 = 6 cal.