Answer:
Option C
Explanation:
For a diamagnetic complex, there should not be any unpaired electron in the valence shell of central metral.In $K_{3}[Fe(CN)_{6}],Fe(III)$ has $d^{5}$- configration (odd electrons). Hence it is paramagnetic. In $[Co(NH_{3})_{6}]Cl_{3},Co(III)$ has d6 configuration in a strong ligand field, hence all the electrons are paired and the complex is diamagnetic .In $Na_{3}[Co(ox)_{3}],Co(III)$ has $d^{6}$- configuration and oxalate being a chelating ligand, very strong ligand and all the six electrons remains paired in lower $t_{2g}$ level. diamagnetic.In $[Ni(H_{2}O)_{6}]Cl_{2},Ni(II)$ has $3d^{8}$ -configuration and $H_{2}O$ ia a weak ligand hence
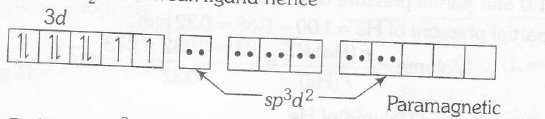
In $K_{2}[Pt (CN)_{4}]Pt(II)$ has $d^{8}$- configuration and $CN^{-}$ is a strong ligand, hence all the eight electrons are spin paired. Therefore, complex is diamagnetic
In $[Zn(H_{2}O)_{6}](NO_{3})_{2},Zn(II)$ has ${_{3}}d^{10}$ configuration spin paired , hence diamagnetic