Answer:
Option B
Explanation:
Plan This problem is based on concept of VBT and magnetic properties of coordination compound.
Draw VBT for each coordination compound
If an unpaired electron is present then the coordination compound will be paramagnetic otherwise diamagnetic.
coordination compounds of [MA4B2] type show geometrical isomerism.
Molecular orbital electrons configuration (MOEC) for various coordination compound can be drawn using VBT as
MOEC for [Cr(NH3)4Cl2]Cl is
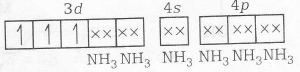
Number of unpaired elecrtons (n)=3
Magnetic properties = paramagnetic
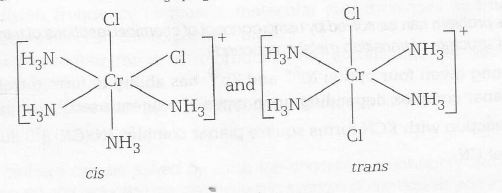
Geometrical isomers of (Cr(NH3)4Cl2]+ are
MOEC of [Ti(H2O)5 Cl](NO3)2 is
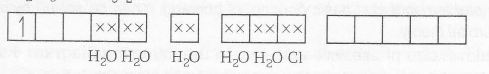
n=1
Magnetic properties= paramagnetic
Ionisation isomers of [Ti(H2O)5Cl)(NO3)2 are [Ti(H2O)5Cl(NO3)2 and [Ti(H2O)5(NO3)]Cl(NO3)
MOEC of [Pt(en)(NH3)Cl]NO3 is
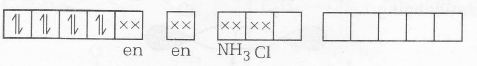
n=0
Magnetic property= diamgnetic
Ionsiation isomers are [Pt(en)(NH3)Cl)NO3 and [Pt(en)(NH3(NO3)]Cl
MOEC of [Co(NH3)4 (NO3)2]NO3 is
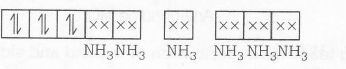
n=0
Magnetic property= Diamagnetic Geometrical isomers are
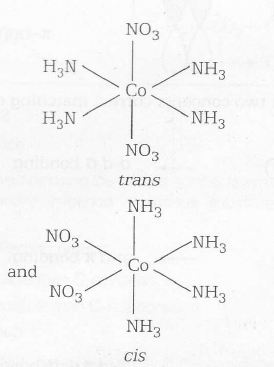
Thus, magnetic property and isomerism in a given coordination compound can be summarised as
(P) [Cr(NH3)4 Cl2]Cl → Paramagnetic and exhibits cis-trans isomerism (3)
(Q) [Ti(H2O)5Cl](NO3)2 → Paramagnetic and exhibits ionisation isomerism (1)
(R) [Pt(en) (NH3)Cl]NO3 → Diamagnetic and exhibits ionisation isomerism(4)
(S) [Co(NH3)4 (NO3)2 ]NO3 → Diamagnetic and exhibits cis-trans isomerism (2)
P → 3, Q→ 1, R→ 4, S→ 2
Hence, (b) is the correct choice